
 |
Ocular Surface Update
Kelly K. Nichols, OD, MPH, PhD, FAAO
|
 |
 |  |  |  |  |
|
This morning I applied my contact lenses like usual and within seconds I could "feel" the lens on my right eye, while my left eye was completely comfortable (no lens awareness). Over the next 30 minutes, the sensation worsened, almost to the point of feeling like foreign body was under the lens. When I arrived at my destination, I removed the lens, checked to see if it was inverted (it wasn't), put a drop of lubricant on it and reinserted it. It was fine, and still was an hour later, but my eye still felt like something happened. There was no visible lash or debris on the lens, no edge tear, or obvious defect.
For me, this pattern occurs about once every 14 days, and most of the time it is not to the level of needing to remove the lens. In some instances, just removing and reinserting the lens helps. In discussions with contact lens manufacturers, I have been told that their studies show that comfort on insertion is often a predictor of ultimate contact lens success, and is viewed as an important factor in developing new lens materials. To my knowledge, though, there is limited published evidence on initial lens comfort as a predictor of dry eye, or other ocular surface disease. There is ample and repeated evidence that the primary factor associated with contact lens dropout is dryness and discomfort, which may or may not be the same thing as comfort (or discomfort) on insertion. I find it particularly interesting that "lens awareness on insertion," either bilateral or perhaps unilateral does not seem to have been as studied in patients with contact lens related dry eye disease. |
 |
Care Solution Corner
Susan J. Gromacki, OD, MS, FAAO
|
 |
 |  |  |  |  |
|
The new Duette Hybrid Contact Lens for Astigmatism (SynergEyes, Inc.) was launched in September 2010, with a broader roll-out in January of this year. It contains a completely different design and novel materials as compared with the original SynergEyes family of lenses. Most notably, the soft skirt in Duette is comprised of Flex2O, a silicone hydrogel, and the central gas permeable portion contains MaxVu, with an increased Dk of 130.
As with the original SynergEyes materials, SynergEyes, Inc. requires daily digital rubbing and rinsing steps with Duette. The company highly recommends Clear Care (CIBA Vision) for disinfection. At the present time, the following multipurpose solutions (MPS) are also approved as the disinfectant for Duette: AQuify (CIBA Vision), Opti-Free Express (Alcon), Opti-Free RepleniSH (Alcon), Complete (Abbott Medical Optics), and ReNu Fresh Lens Comfort (formerly ReNu MultiPlus, Bausch + Lomb). All of these MPS may also be utilized for the digital rubbing step; for heavy depositors, a separate daily cleaner approved for use with soft contact lenses is recommended. It is important to note that gas permeable (GP) contact lens cleaning and disinfection solutions are contraindicated with Duette. The company recommends replacing the lenses every six months.
|
|


 |
Allergy Sufferers Contend with Longer Allergy Season
The 2011 allergy season is expected to be 27 days longer in northernmost parts of North America, adding almost a month of suffering to the typical pollen allergy season of February/March-October, according to a study published by the Proceedings of the National Academy of Sciences.
The longer allergy season could be particularly rough on eye allergy sufferers, as ocular allergies affect one in every five individuals and it is estimated that 50 percent of individuals with seasonal and indoor allergies also experience some degree of ocular allergy.1,2
To help allergy sufferers better understand and manage their condition, the Asthma and Allergy Foundation of America (AAFA) offers a free educational brochure, Eye Health and Allergies. The brochure, which also includes smart allergy season strategies for contact lens wearers, can be viewed or downloaded at www.aafa.org/eyeallergies.
1.Katelaris CH, Bielory L. Evidence-based study design in ocular allergy trials. Curr Opin Allergy Clin Immunol. 2008;8(5):484-488.
2.Bassett C. Ocular Allergies. Asthma & Allergy Advocate. Summer 2007. American Academy of Allergy Asthma & Immunology Web site. www.aaaai.org/patients/advocate/~. Accessed November 3, 2008.
| -- ADVERTISEMENT -- |
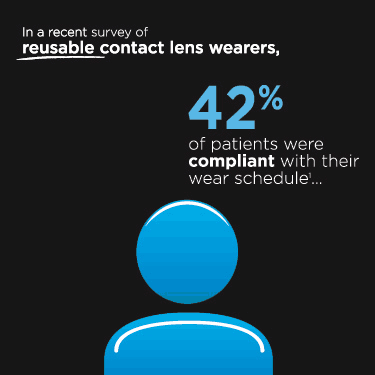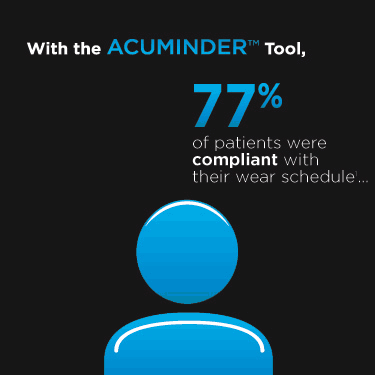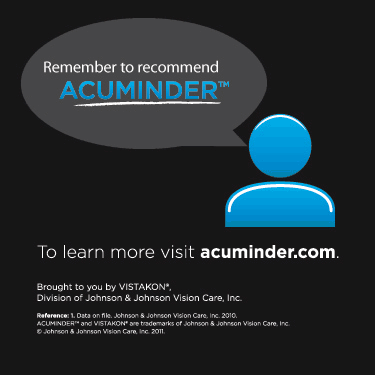
|
Contact Lens Clinical Pearls Pocket Guide
Now Available
The GP Lens Institute (GPLI) announced the availability of the Contact Lens Clinical Pearls Pocket Guide, a valuable resource for all eyecare practitioners who fit contact lenses, whether or not they currently fit GP lenses.
The Contact Lens Clinical Pearls Pocket Guide is a helpful tool to have readily available when fitting or evaluating a spherical GP or specialty contact lens patient. This 24-page resource offers concise clinical pearls pertaining to the fitting of spherical GP, soft toric, GP back surface and bitoric, soft and GP multifocal, orthokeratology, keratoconus, and scleral lens designs. Diopter-to-radius conversion and vertex conversion charts are also provided.
The Contact Lens Clinical Pearls Pocket Guide may be downloaded at www.gpli.info/pdf/pearl-fitting-guide.pdf or the pamphlet may be ordered at www.gpli.info.
April Contact Lens Spectrum
The cover story in this month's issue of Contact Lens Spectrum presents a Specialty Contact Lens Update. In this article, Gretchyn M. Bailey, NCLC, FAAO reviews of some highlights from the 2011 Global Specialty Lens Symposium.
Other feature articles include: Microbiology and Contact Lens Wear by Mark D.P. Willcox, PhD and Contact Lens-Associated Infiltrative Keratitis and Multipurpose Solutions by Andrew J. Sacco, OD, FAAO.
Look for your issue in the mail to read these and other informative articles or review issue on line at: www.clspectrum.com/thismonth.aspx.
--ADVERTISING
RegeneRx Focuses Clinical Development on Dry Eye Indication with RGN-259
RegeneRx Biopharmaceuticals, Inc. announced that it has shifted its near-term clinical development focus to RGN-259, its preservative-free topical eye drop, for the treatment of symptomatic dry eye. This reprioritization of the Company's product pipeline is primarily due to recently announced data supporting the development of RGN-259 for this indication.
According to the company, in two studies evaluating RGN-259 to treat symptomatic dry eye induced in mice, RGN-259 resulted in statistically significant improvement of corneal healing compared with negative and positive controls. Previous studies in animal models have also shown the ability of RGN-259 to repair the cornea after chemical damage. Following these animal data, and in conjunction with previously reported human clinical data, RegeneRx is planning a Phase 2 clinical trial in patients with dry eye that will be designed to measure the safety and efficacy of RGN-259 in this indication. This will be in addition to a physician-sponsored clinical trial in patients with dry eye that is currently being supported by RegeneRx in the form of manufacturing and regulatory and clinical guidance.
Study Finds Employers Offering Vision Benefits Save $4.5 billion on Healthcare Expenditures
VSP Vision Care released the findings of a new study showing $4.5 billion in savings for its clients – for profits, not-for-profits and government organizations – through the early detection of chronic diseases via eyecare and vision exams covered by VSP vision insurance.
The study, conducted by Human Capital Management Services Group (HCMS), a national human capital consulting firm, found that for every $1 invested in VSP exam services, which include comprehensive, annual eye exams, during an employee's first year with the benefit, employers can expect an average two-year total return of $1.27 in long-term healthcare savings. These savings are a result of avoided medical costs and increased employee productivity.
Further, according to the study, VSP clients experienced 7% less absenteeism, 4% less employee turnover and savings on insurance and workers' compensation costs. Early detection of chronic diseases like diabetes and hypertension also increased the likelihood employees would be proactive with their healthcare and more likely to see a medical doctor to receive follow-up care.
Individuals who have a VSP vision plan are three times more likely to get an annual eye exam than a routine preventive physical, per the company, which means VSP providers are more likely to detect the first signs of common chronic diseases like diabetes and hypertension.
To view this study, visit vspeffect.com.
This month at www.siliconehydrogels.org: Ethnic differences in ocular physiology, tear mixing and contact lens-related adverse events, risk factors for inflammatory and mechanical events, and our synopsis of the 2010 meeting of the American Academy of Optometry.
|
 |
 |
|

 |
Editor's Commentary
Jason J. Nichols, OD, MPH, PhD, FAAO
|
 |
 |  |  |  |  |
|
Please see this week's Quick Poll results, which I once again find very fascinating. For many years, the field has been quite focused on the importance of oxygen permeability, albeit more likely as it relates to other safety factors. However, over the last 10 years, the role of oxygen permeability in contact lens related dry eye has also been discussed, with some suggesting it is quite important for many years now. However, it seems as though, based on our results, that the perceptions of practitioners may indeed be changing in this regard. Deposit resistance and friction (or lack thereof) seem to be on today's practitioners minds.
Look for more on these issues in our annual dry eye issue of Contact Lens Spectrum, which prints in July. |
|
CLToday Quick Poll
|
|
Last week's question:
When refitting a soft lens patient with contact lens dry eye and reduced comfortable wearing time, which contact lens material characteristic do you consider most important to aid in alleviating their dryness and discomfort?
1. Deposit resistance
 32% 32%

2. Friction (slipperiness)
 33% 33%

3. Modulus (stiffness)
 14% 14%
4. Oxygen permeability
 21% 21%

|
|
Abstract
|
|
Biomedical Soft CL Sensor for In Situ Ocular Biomonitoring of Tear Contents
A soft contact-lens biosensor (SCL-biosensor) for novel non-invasive biomonitoring of tear fluids was fabricated and tested.
Wearing a biosensor on eye enabled the in situ monitoring of tear contents. The biosensor has an enzyme immobilized electrode on the surface of a polydimethyl siloxane (PDMS) contact lens. The SCL-biosensor was fabricated using microfabrication techniques for functional polymers (PDMS and 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer).
In investigation of in vitro characterization, the SCL-biosensor showed excellent relationship between the output current and glucose concentration from 0.03 to 5.0 mmol•L(-1), with a correlation coefficient of 0.994. The calibration range covered the reported tear glucose concentrations (0.14 mmol•L(-1)).
Based on the result, ocular biomonitoring with the SCL-biosensor was carried out. The SCL-biosensor well worked both in the static state and the dynamic state. The tear glucose level of rabbit was estimated to 0.12 mmol•L(-1) at first and then the tear turnover was successfully calculated to be 29.6 +/- 8.42% min(-1).
According to the researchers, the result indicated that SCL-biosensor is useful for advanced biomonitoring on eye.
Chu M, Shirai T, et al. Biomedical soft contact-lens sensor for in situ ocular biomonitoring of tear contents. Biomed Microdevices 2011. Apr 8. [Epub ahead of print]
|
|











