
-- ADVERTISEMENT --
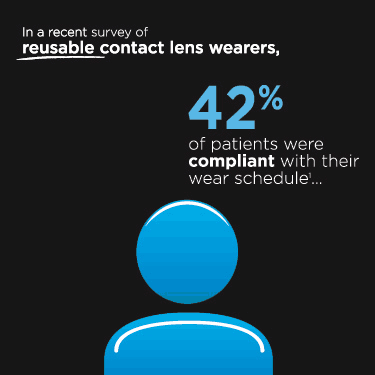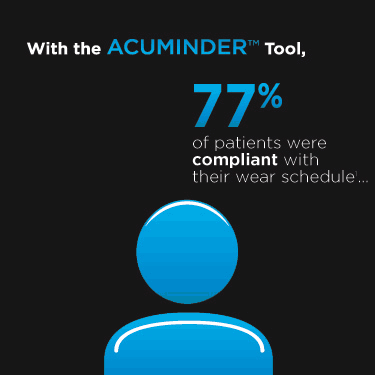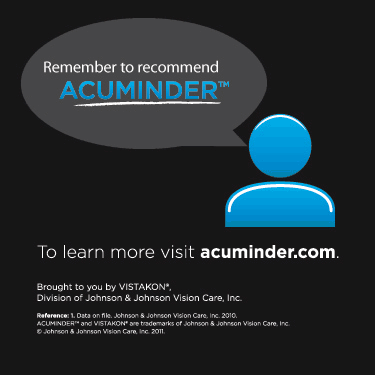

If you are having problems voting, your email settings may be blocking you. Click here to vote through your browser.
|
|

|
I have many good colleagues who feel quite strongly about diagnostic fitting with nearly all GP contact lens fits. However, I also realize that there are many practitioners who usually go the empirical route. I think it's important for students and practitioners to learn and understand the diagnostic fitting process first, but ultimately I feel it is important to do what's right for the patient and the specific situation. GP labs have excellent support staff who can always help you along the way.
|

Lensfindr, LLC announces the new contact lens information website for the eyecare professional, www.lensfindr.com. The web based software program will allow the contact lens clinician to quickly search and select specific soft contact lens information for their patients.
There are multiple fields that allow a customized search process that is quick, accurate, and specific. According to the company, the database will include lenses primarily from Bausch + Lomb, CIBA Vision, CooperVision and Vistakon. A select number of specialty contact companies will be included and revised as per demand by the users. The site will also feature other useful contact lens information including a manufacturer's directory, solution charts, rigid gas permeable lens information, contact lens products, and industry/news updates.
For more information or to subscribe to the site, eyecare professionals can visit www.lensfindr.com. |
Bausch + Lomb (B+L) is conducting a voluntary recall of a single lot of Murocel Methylcellulose Lubricant Ophthalmic Solution, USP 1%, an over-the-counter ophthalmic solution indicated for the temporary relief of burning and irritation due to dryness of the eye. This single lot recall is limited only to the United States; it does not affect locations in Europe, the Middle East or Asia-Pacific regions.
B+L chose to initiate this recall based on routine stability testing which showed the single lot was out of specification for preservative efficacy. There have been no adverse events reported which have been attributed to the single product lot being out-of-specification for preservative efficacy. B+L is recalling the single lot of product in the interest of patient safety.
B+L has directly contacted U.S. retailers who have been shipped this product to initiate the recall and also contacted eye care professionals to alert them of the recall. Consumers who have affected product should call the B+L customer service center for instructions on returning and reimbursement: 1-800-553-5340.
Affected Product Details: Lot Number: 507521, UPC Code: 3 24208 28015 7 and Expiration Date: 1-31-2012 |

Plan now to attend the Global Specialty Lens Symposium to be held January 26 - 29, 2012 at the Paris Hotel and Casino in Las Vegas, Nevada. This meeting will include insightful presentations by international experts in the field, hands-on demonstrations of cutting-edge products and valuable continuing education credits.
|
The Program Committee of the GSLS invites the submission of Papers and Posters. Papers and abstracts related to presbyopia, keratoconus, corneal topography, post penetrating keratoplasty or related irregular corneal surface, myopia control, orthokeratology and lens care topics are welcome.
New this year is the photo contest. Contestants may submit up to two (2) photographic images in the following anterior segment categories: Contact Lens, Lids, and Cornea/Conjunctiva. Contestants may also submit images obtained utilizing such equipment as OCT, topographers, etc.
Those interested in submitting may visit www.GSLSymposium.com for more information. Web submissions only. Deadline for submissions is August 31, 2011.
--ADVERTISING |
|
The Blanchard Contact Lens Research and Professional Development Center in Manchester, New Hampshire hosted its inaugural session featuring the msd Mini Scleral Design in June.
The Center was designed to provide eye care practitioners up-to-the-minute fitting techniques in a small group setting. Hands-on, practical learning, joined with state-of-the-art digital imaging equipment, provided attendees with a high quality learning experience. The session was limited to eight attendees in order to maintain the quality of the session and hands-on experience.
Session agenda included: a tour of the Blanchard laboratory and lens production; in depth presentation of the msd lens with Stephen Byrnes, OD, who has fit well over 100 patients with the msd lens; a hands-on workshop, learning and practicing techniques for applying and removing lenses in the wet lab with Dr. Byrnes; and active participation in a series of patient fits with Dr. Byrnes.
Blanchard plans to host additional sessions in August, September, and October. Those wishing to attend should contact their Blanchard Sales Representative to discuss availability. For more information visit www.blanchardlab.com or call 800 367-4009.
|
|
Bausch + Lomb announced plans to launch a pharmaceutical business in India through a strategic agreement with Micro Labs. The partnership will provide B+L with high-quality manufacturing capabilities in the region which will speed the introduction of new prescription and over-the-counter medicines targeted at a wide range of eye diseases.
Through the agreement with Micro Labs, B+L aims to capture part of the rapidly expanding ophthalmic pharmaceuticals market in India which is expected to reach US$300 million by 2015. B+L will also establish dedicated sales and marketing teams, and deliver practitioner and patient education programs designed to improve the quality of, and access to, eye care.
The companies will introduce up to six new pharmaceuticals eye drops including Moxisurge, Aquasurge, Aquasurge Max, Bromvue, Ketovue and Moxisurge-KT. The companies have also agreed to explore other areas of possible collaboration including:
- Sourcing of Ophthalmic Solution products from Micro Labs to be marketed and sold by B+L in India and other markets in Asia Pacific.
- Collaboration between the companies with respect to manufacturing technology for the production of eye drops.
- Joint research and development of Ophthalmic Pharmaceutical products specifically for emerging markets.
|
|
Prevent Blindness America (PBA) is planning the fourth annual Swing Fore Sight Golf Tournament. The event will be held in conjunction with Vision Expo West on Sept. 21, 2011 at the Bali Hai Golf Club in Las Vegas, Nevada. The goal of the event is to raise $140,000 to support the sight-saving programs of Prevent Blindness America.
Transitions Optical, Inc. will once again be serving as the presenting sponsor. Additional sponsors of the Swing Fore Sight event include Advantica, Essilor, Gilbert Displays, Hilco, Hoya Vision Care, Kenmark, National Association of Vision Care Plans (NAVCP), Refac Optical Group, Stereo Optical, UVEX by Honeywell, Vision-Ease, Viva International and Zyloware.
Individual golfers may sign up for $625 or foursomes for $2,750. The tournament is a four-person scramble. Participants may register online at preventblindness.org/swingforesight.
^ Back to top |

Epithelial Microcysts
Gregory W. DeNaeyer, OD, FAAO
|
|  |
|
 This photograph shows midperipheral epithelial microcysts. The patient had been wearing his -1.50D OD and -1.75D OS CIBA Vision Night & Day lenses on an extended wear basis. He reported removing and sleeping without his lenses once or twice a week. The patient replaced his lenses on a monthly basis. His visual acuity was 20/20 in both eyes, and he was asymptomatic. This photograph shows midperipheral epithelial microcysts. The patient had been wearing his -1.50D OD and -1.75D OS CIBA Vision Night & Day lenses on an extended wear basis. He reported removing and sleeping without his lenses once or twice a week. The patient replaced his lenses on a monthly basis. His visual acuity was 20/20 in both eyes, and he was asymptomatic.
For more on this patient, see http://www.clspectrum.com/article.aspx?article=&loc=archive\2009\april\cls_april_a02.html.
We welcome photo submissions from our readers! It is easy to submit a photo for consideration for publishing in Contact Lenses Today. Simply visit http://www.cltoday.com/upload/upload.aspx to upload your image. Please include an explanation of the photo and your full name, degree or title and city/state/country.
|

 |  |
 | RESEARCH REVIEW
Loretta B. Szczotka-Flynn, OD, PhD, MS, FAAO |
 |  |
 |  |
|
Pregnancy and Keratoconus
There has long been the thought that hormonal changes that accompany pregnancy can influence the progression (or occurrence) of keratoconus. However, there are no good case reports in the literature to support this. I have at least one patient that fits this category.
A new paper reports a case series of four patients that showed progression of keratoconus during pregnancy, excluding patients with accompanying systemic and ocular diseases associated with keratoconus, uncontrolled atopic disease, and eye rubbing. Bilgihan and colleagues in Turkey documented seven eyes of four pregnant women that showed changes in refraction, corneal topography, and rigid gas-permeable lens fitting pattern during pregnancy.
The mean age of patients was 29.3 years. Mean increase in spherical equivalent refraction and simulated keratometry values were 1.4 ± 1.1 and 1.1 ± 0.8 diopters, respectively. In eyes wearing rigid gas-permeable lenses, increase in corneal apical touch and decrease in the base curve radius of the best-fitting contact lens were observed. This is probably the first study showing pregnancy-induced keratoconus progression in patients with no other accompanying disease or risk factor for keratoconus.
Bilgihan K, Hondur A, Sul S, Ozturk S. Pregnancy-induced Progression of Keratoconus. Cornea. 2011 Jun 24. [Epub ahead of print]
^ Back to top
|
 |  |
 | MATERIALS & DESIGNS
Ronald K. Watanabe, OD, FAAO |
 |  |
 |  |
|
A New Way to Keep Lenses Wet
In this column, I have discussed various methods that have been developed to improve comfort of contact lens materials. These include wetting agents bound within the lens matrix, wetting agents in the blisterpack, and polymer chemistry that increases water binding properties. In addition, new lens care products have been developed that improve surface wettability and deposit resistance. There are also numerous rewetting drops that can temporarily improve surface wetting during lens wear.
Researchers at Auburn University have published a paper on a silicone hydrogel lens they have modified to release a wetting agent over the course of several days. The paper describes a molecular imprinting process that can be modified to allow the release of hydroxypropyl methylcellulose for up to 60 days while the lens is worn on a continuous wear basis. They report good optical and mechanical properties of the modified lens. This may be a future way to improve long-term comfort, and possibly even as a drug delivery device for chronic eye conditions. We look forward to more developments like this in the quest for comfort.
White CJ, McBride MK, Pate KM, et al. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials 2011; 32(24): 5698-5705.
^ Back to top
|

A Histopathological Study of Bulbar Conjunctival Flaps Occurring in Two Contact Lens Wearers
|
Researchers wanted to study the histopathology of paralimbal bulbar conjunctival flaps occurring secondary to soft contact lens wear.
Slit-lamp biomicroscopy using sodium fluorescein, cobalt blue light, and a Wratten filter was used to observe the presence, location, and dimensions of bulbar conjunctival flaps presenting in a cohort of contact lens wearers. Two subjects who exhibited such flaps agreed to undergo conjunctival biopsy. Tissue samples, obtained from the region of the flap, and an adjacent unaffected area were processed by standard histopathological methods.
In the first subject, analysis of the flap tissue showed even collagen distribution and overall normal histology. The flap of the second subject displayed a mild focal increase in collagen and mild degeneration of collagen, but no increase in elastic tissue. Conjunctival epithelium was normal in both cases.
The authors concluded that in these two subjects conjunctival flap tissue either was normal or showed only minimal abnormality. There is insufficient evidence for significant pathological change on the time scale of this study.
Markoulli M, Francis IC, Yong J et al. A Histopathological Study of Bulbar Conjunctival Flaps Occurring in 2 Contact Lens Wearers. Cornea. 2011 Jun 28. [Epub ahead of print]
^ Back to top |
|
|
 |
This month at www.siliconehydrogels.org:
Ethnic differences in ocular physiology, tear mixing and contact lens-related adverse events, risk factors for inflammatory and mechanical events, and our synopsis of the 2010 meeting of the American Academy of Optometry.
Important Links:
To report adverse contact lens reactions visit: http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers Act or associated Contact Lens Rule visit: https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe; Unsubscribe; Submit Clinical Image
Submit news to news@cltoday.com.
Send your comments and fitting tips to cltoday@wolterskluwer.com. Please include your full name, degree or title and city/state/country.
For more information on Contact Lenses Today including archives of previous issues, please visit our website at www.cltoday.com. For the latest articles on contact lenses, important clinical information and helpful tools related to the contact lens practice visit the Contact Lens Spectrum website at www.clspectrum.com.
Contact Lenses Today and CLToday are registered trademarks of:
Wolters Kluwer Pharma Solutions VisionCare Group, 323 Norristown Road, Suite 200, Ambler, PA 19002 | 215-646-8700
© 2011 All Rights Reserved
|
 |
|
|















 This photograph shows midperipheral epithelial microcysts. The patient had been wearing his -1.50D OD and -1.75D OS CIBA Vision Night & Day lenses on an extended wear basis. He reported removing and sleeping without his lenses once or twice a week. The patient replaced his lenses on a monthly basis. His visual acuity was 20/20 in both eyes, and he was asymptomatic.
This photograph shows midperipheral epithelial microcysts. The patient had been wearing his -1.50D OD and -1.75D OS CIBA Vision Night & Day lenses on an extended wear basis. He reported removing and sleeping without his lenses once or twice a week. The patient replaced his lenses on a monthly basis. His visual acuity was 20/20 in both eyes, and he was asymptomatic.


