|
I've noticed more stories lately in the general media about the illegal selling of contact lenses. When done right, I think this coverage is helpful in getting the message out that contact lenses are medical devices to be dispensed by an appropriate eyecare practitioner. With some exception, they should not be slept in, and require care and disposal at the appropriate time. Continued diligence on our part is also critical in this process. |

Newton K. Wesley, OD, MD, ScD, PhD died July 21 of congestive heart failure in Freeport, Illinois. Dr. Wesley was a contact lens pioneer, lecturer, writer, educator, clinician and inventor. He created solutions for his keratoconus that led to his development and manufacturing of contact lenses and is often cited for inventing the first commercially successful rigid contact lens in the 1950s.
Dr. Wesley was born Newton Uyesugi to immigrant Japanese parents in Oregon. In 1939, he graduated from North Pacific College of Optometry. At 22 he had an optometric practice in Portland, Oregon, and partnered with Dr. Roy Clunes to buy and operate his alma mater which is now the Pacific College of Optometry.
During World War II, he and his family interned in the Minidoka Relocation Camp in Idaho. Wesley, who for business purposes anglicized his name to what he thought sounded similar to his Japanese last name, received permission to leave the camp to study chemistry at Earlham College in Indiana, though his family remained at the camp. At the end of the war, he relocated his family to Chicago, where he saw patients and taught at Monroe College of Optometry, today the Illinois College of Optometry.
Since his youth Wesley had searched for a cure for his worsening vision. He found that large molded glass contact lenses restored normal sight but were almost intolerable to wear for more than a short period of time. In Chicago, he began working on the development of smaller plastic contact lenses with his former optometry student, George Jessen. Together they formed the Plastic Contact Lens Company (1946), which became Wesley-Jessen, Inc., which was bought by Schering Plough in 1980 and is now owned by CIBA Vision. They also formed the Jessen-Wesley eye clinic in Chicago.
Dr. Wesley founded the National Eye Research Foundation. He helped get the Doctor of Optometry degree recognized nationally and was instrumental in getting the words "contact lenses" into the dictionary. Wesley even learned to fly a plane in order to make his training on contact lenses available to as many doctors as he could. He also trained doctors in Europe and Asia.
Memorial services will be held on August 20th at the Anderson Japanese Gardens in Rockford, Illinois. |
RegeneRx Biopharmaceuticals, Inc. announced that it is set to begin a Phase 2 clinical trial in 72 patients with dry eye syndrome. The Company anticipates enrollment of the first patients on August 13th, and expects preliminary data from the study to be available in October 2011. The trial is a double-masked, placebo-controlled trial testing the safety and efficacy of RGN-259, the Company's proprietary preservative-free eye drops, against a placebo. Patients will receive RGN-259 or placebo twice daily for 30 days. Signs and symptoms of dry eye, such as the degree of ocular surface damage, ocular itching, burning and inflammation, among others, will be measured periodically during the treatment period. The trial will be conducted by ORA Inc., an ophthalmic contract research organization that specializes in dry eye research and clinical trials. Additional details regarding the Phase 2 trial may be seen online at:
www.clinicaltrials.gov/ct2/show/NCT01387347?term=thymosin+beta+4&rank=5. |
RGN-259 is a sterile, preservative-free topical eye drop for ophthalmic indications. RegeneRx has recently completed two animal studies showing RGN-259's positive effects on dry eye symptoms and has positive human data indicating its ability to heal corneal ulcers. RegeneRx is currently also supporting a small physician-sponsored Phase 2 dry eye study with RGN-259. |

August 31, 2011 is the deadline for submission of papers and posters for the Global Specialty Lens Symposium (GSLS), to be held January 26 - 29, 2012 at the Paris Hotel and Casino in Las Vegas. Papers and abstracts related to presbyopia, keratoconus, corneal topography, post penetrating keratoplasty or related irregular corneal surface, myopia control, orthokeratology and lens care topics are welcome.
New this year is the photo contest. Contestants may submit up to two (2) photographic images in the following anterior segment categories: Contact Lens, Lids, and Cornea/Conjunctiva. Contestants may also submit images obtained utilizing equipment such as OCT, topographers, etc.
Visit www.GSLSymposium.com for more information. Web submissions only.
--ADVERTISING
|
Nine schools and colleges of optometry have received diversity mini-grants through the Association of Schools and Colleges of Optometry's (ASCO) 2011 Optometric Education Diversity Mini-Grant Program. This program is supported by Luxottica Retail and The Vision Care Institute, LLC.
The grants are designed to provide seed money for a specific program/project to assist schools/colleges of optometry with their long-term diversity/multicultural efforts. Programs may include, but are not limited to, summer bridge programs for undergraduate students, mentoring and guidance programs for first-year optometry students and other programs to promote optometry as a career among underrepresented groups.
The following diversity programs were selected: Illinois College of Optometry - Focus on Your Future Summer Program; The Ohio State University College of Optometry - Eye Care for All; Pacific University College of Optometry - Pacific University InSight 2011; Southern College of Optometry - Success in Sight; SUNY State College of Optometry - Increasing Diversity by Engaging All (IDEA); University of Alabama at Birmingham School of Optometry - Providing Diversity in Optometric Education through Continual Enhancement of Current Programs that Promote Diversity in Optometry; University of California, Berkeley School of Optometry - Berkeley Optometry Opto-Camp; University of Houston College of Optometry - Optometry Career Opportunities Program (TEXOCOP); and Western University of Health Sciences College of Optometry - Reaching Out to Families and Communities - Opening Eyes to Optometry.
^ Back to top |

|
Patient Recall of Contact Lens Products
|
|  |
|
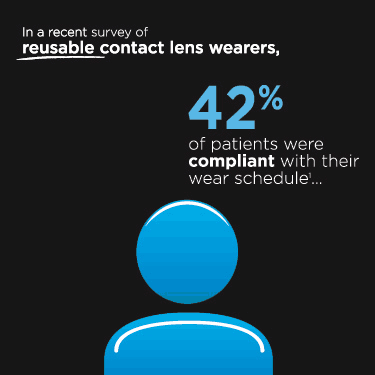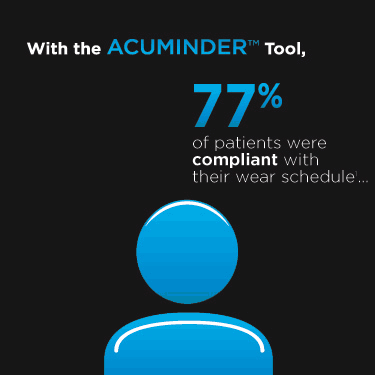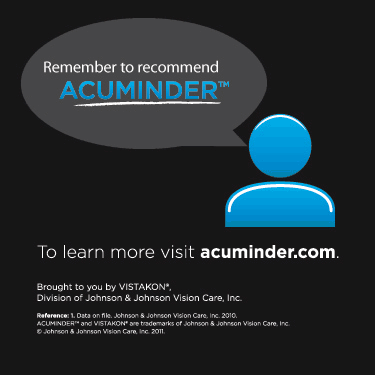
I am writing in regards to the abstract titled "Ability of Patients to Recall Habitual Contact Lens Products" published in Contact Lenses Today, July 24, 2011 edition. Dr. Dumbleton and colleagues found that 51% of patients could recall their lens products and 41% recalled their lens care system both by memory. In my clinical experience, lens memory recall is probably much lower in eyecare offices (in my opinion maybe 25-35%), because most patients are still purchasing lenses directly from the doctor's office, so their conscious purchasing decision to choose a brand is quite low.
I would tend to think lens care memory recall would slightly be higher than actual lenses (in my opinion maybe 35-40%) since this is a consumer purchase at every U.S. retail pharmacy outlet. Even though we strive to 'proscribe' a lens care solution in our office, the patient still compares products at the retail aisle thus possibly increasing memory recall. I would like to commend Dr. Dumbleton and colleagues for their efforts and encourage them or any other research facility to conduct a similar study with more subjects for verification.
Michael Mayers, OD, FAAO
Powell, Ohio |

 |  |
 |
CARE SOLUTION CORNER
Susan J. Gromacki, OD, MS, FAAO |
 |  |
 |  |
|
New Product: Opti-Free PureMoist
In this January's Contact Lens Spectrum, Editor-in-Chief Jason Nichols called 2010 the "Year of the Contact Lens Solution."
(http://www.clspectrum.com/article.aspx?article=105075) He selected as the "Contact Lens Event of the Year" the launch of two new multipurpose contact lens care solutions, with the addition of "at least one more" contact lens care product in 2011. That product is now available.
Alcon introduced Opti-Free PureMoist Multipurpose Disinfecting Solution this month. It was designed to improve upon the market leader (Opti-Free RepleniSH). Like RepleniSH, it was developed for use with both silicone and traditional hydrogels and it utilizes the cleaning agents Citrate and Tetronic 1304.
There are several differences between PureMoist and RepleniSH. Alcon remained with its highly successful combination of preservatives/active ingredients, Polyquaternium-1 (Polyquad) and Aldox. However, it increased the concentration of Aldox (from 0.0005 to 0.0006%) and added the chelating agent ethylene diamine tetraacetic acid (EDTA) to enhance disinfection.
PureMoist's breakthrough technology is in its novel wetting agent, poly(oxyethylene)-poly (oxybutylene) (HydraGlyde Moisture Matrix). Poly(oxyethylene) is hydrophilic; it attracts moisture and carries water to the hydrophobic lens sites not wetted by the tear film. The poly(oxybutylene) chains target silicone, surrounding its hydrophobic groups and rendering the surface wettable. The ultimate goal is increased comfort and wearing time for our lens-wearing patients.
^ Back to top
|
 |  |
 |
OCULAR SURFACE UPDATE
Kelly K. Nichols, OD, MPH, PhD, FAAO |
 |  |
 |  |
|
Dietary Omega-3 Fatty Acids
Recently I have been thinking about dietary recommendations of omega fatty acids for dry eye or meibomian gland disease. There have been a few open label studies of omega supplements for dry eye, yet the "modern" dietary recommendations for dry eye or MGD have been poorly elucidated. We all think our dietary consumption of omega fatty acids is adequate, but do we really stop to consider what the nutritional guidelines recommend?
Certainly it is widely known that some fish are an abundant source, but did you know butter or margarine can be as well? Certain brands of butter contain canola oil to aid spreading, providing up to "10% of the daily recommendation" of omega fatty acids (ALA). It is possible that a dry eye patient could consume significant amounts of omega-3 via butter or margarine (with canola oil, for example)? This makes the discussion a bit more challenging—what if a patient consumes on average 2 grams of omega-3 per day via margarine, on food?
This point demonstrates that the omega fatty acid story is far from complete, yet remains an exciting avenue for clinical research in ocular surface disease.
^ Back to top
|

OCT Imaging of Lid-Parallel Conjunctival Folds in Soft Contact Lens Wearers
|
|
Researchers' goal was to visualize, using Fourier-domain optical coherence tomography (FD-OCT), the alterations in lid-parallel conjunctival fold (LIPCOF) morphology in eyes with contact lenses and after their removal.
One randomly selected eye of each of 24 volunteers with normal ocular surface status was examined using FD-OCT. The study group included 18 female and 6 male subjects, with a mean age of 28.9 years (range, 18-50 years). The presence, height, and number of conjunctival folds and the tear meniscus area were evaluated by FD-OCT, and the difference between "before and after lens removal" were analyzed by the Wilcoxon signed-rank test. To determine the coverage of the folds, the OCT-LIPCOF grading system was applied. Visualization of the folds was compared on linear and raster scans. The Spearman rank test was applied for correlations (SPSS 16.0; SPSS Inc., Chicago, MI).
OCT scans were able to visualize and objectively describe the LIPCOF and its relation to the tear film in contact lens wearers. Raster scanning provided more information about the folds, but the measurements were easier to carry out on the single line scans. After removal of the contact lens, the height of the conjunctival folds significantly decreased (p < 0.001), and the tear meniscus area significantly increased (p = 0.017). The number of LIPCOF did not change (p = 0.971), but LIPCOF coverage by the tear film increased after lens' removal and resulted in changes in the OCT-LIPCOF grading (p < 0.001).
The researchers concluded that FD-OCT could be a quick, non-invasive, quantitative method for the imaging of LIPCOF in contact lens patients. Also, when grading LIPCOF, the mechanical forces of the lens and the tear meniscus changes caused by the lens should be taken into account as these factors influence results. Follow-up of the patients using the same methods is suggested with or without contact lenses.
Tapaszto B, Veres A, Kosina-Hagyo K, Somfai GM, Nemeth J. OCT Imaging of Lid-Parallel Conjunctival Folds in Soft Contact Lens Wearers. Optom Vis Sci. 2011 Jul 8. [Epub ahead of print]
^ Back to top |
|
|
 |
Important Links:
To report adverse contact lens reactions visit: http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers Act or associated Contact Lens Rule visit: https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe; Unsubscribe; Submit Clinical Image
Submit news to news@cltoday.com.
Send your comments and fitting tips to cltoday@wolterskluwer.com. Please include your full name, degree or title and city/state/country.
For more information on Contact Lenses Today including archives of previous issues, please visit our website at www.cltoday.com. For the latest articles on contact lenses, important clinical information and helpful tools related to the contact lens practice visit the Contact Lens Spectrum website at www.clspectrum.com.
Contact Lenses Today and CLToday are registered trademarks of:
Wolters Kluwer Pharma Solutions VisionCare Group, 323 Norristown Road, Suite 200, Ambler, PA 19002 | 215-646-8700
© 2011 All Rights Reserved
|
 |
|
|