

We are seeing the term “biomarker” being used more and more frequently as it relates to the front of the eye and ocular surface disease. Technically this term relates to a “biological marker” that relates to disease status or a condition. Our hope is to not only have a biomarker for ocular surface disease, but perhaps one that will allow us to gauge and predict successful and safe contact lens wear.
|

|
Unilens Vision Inc. and Diversified Ophthalmics, Inc. announced that Diversified has been named an authorized distributor throughout the United States for
Unilens' entire C-Vue family of disposable and C-Vue Advanced Custom contact lens brands. The lenses are available for sale now through all thirteen
Diversified Ophthalmic locations.
C-Vue disposable brand product offerings available now include the C-Vue Aspheric Single Vision and 1-Day options, the original C-Vue Multifocal (in
polymacon) and the new disposable C-Vue ADDvantage Multifocal for Presbyopia contact lens in silicone hydrogel material for monthly replacement. The C-Vue
ADDvantage incorporates Unilens' highly advanced next-generation multifocal contact lens design technology, which allows for ease of fit by providing a
consistent near ADD power across all power profiles, ultimately resulting in clear vision for presbyopes at all distances. The lens is also thinner and
more rounded at the edges for comfort.
With offices and labs from Spokane, Washington to Columbia, South Carolina and from Milwaukee, Wisconsin to Houston, Texas, Diversified supplies eye care
practices in all 50 states. Diversified Ophthalmics, now the 11th largest independently owned laboratory in the U.S., remains focused on being the Single
Source supplier with the quality products, great service, and the reasonable pricing needed by independent eye care practices.
For information on the companies and their products and services, visit their websites, www.unilens.com and www.divopt.com.
|
|
In keeping with its commitment to making a positive impact on local communities, ABB Optical Group has launched a signature annual grants program to
support non-profit organizations nominated by eye care professionals nationwide.
In its inaugural year, the ABB Cares program will highlight and celebrate outstanding organizations throughout the country that have improved the quality
of life in their communities. Five organizations will be selected to receive a $1,000 grant.
Organizations do not need to focus on eye health to qualify. Applications are now available and must be completed and submitted online at http://www.abbconcise.com/ABBOpticalCares by Friday, September 12.
For more information, please contact Nan Callan-Zamora, ABB Corporate Communications and Events Administrator, at 888-305-3300 ext. 7964 or nan@abboptical.com.
|
 Plan now to attend the Global Specialty Lens Symposium to be held January 22 – 25, 2015 at Bally's Hotel and Casino in Las Vegas, Nevada. This meeting will
include insightful presentations by international experts in the field, hands-on demonstrations of cutting-edge products and valuable continuing education
credits.
Plan now to attend the Global Specialty Lens Symposium to be held January 22 – 25, 2015 at Bally's Hotel and Casino in Las Vegas, Nevada. This meeting will
include insightful presentations by international experts in the field, hands-on demonstrations of cutting-edge products and valuable continuing education
credits.
The Program Committee of the GSLS invites the submission of Papers and Posters. Papers and abstracts related to presbyopia, keratoconus, corneal
topography, post penetrating keratoplasty or related irregular corneal surface, myopia control, orthokeratology and lens care topics are welcome.
To submit a photo for the photo contest, submit up to two (2) photographic images in the following anterior segment categories: Contact Lens and
Cornea/Conjunctiva/Lids. Contestants also will be able to submit images obtained utilizing such equipment as OCT, topographers, etc.
Visit www.GSLSymposium.com for more information. Web submissions only. Deadline for submissions is August 31, 2014.
--ADVERTISING
|
|
On August 15, 2014, the FDA’s Center for Devices and Radiological Health granted a 1-year extension of the Unique Device Identification System (UDI)
compliance date for Class III contact lens and intraocular lens labelers. These devices include: extended wear RGPs, overnight orthokeratology lenses,
extended wear soft (hydrophilic) lenses, intraocular lenses including multifocal, accommodative, torics, and phakic, and iris reconstruction lenses.
On September 24, 2013, the FDA published a final rule establishing a unique device identification system (the UDI Rule). The UDI Rule outlines labeling,
data submission and standard date formatting requirements for all medical devices in commercial distribution in the U.S., unless an exception or
alternative applies. The rule will be phased in over a 7-year period through an established set of compliance dates. The compliance date for class III
devices is September 24, 2014.
Recently, certain labelers of the contact lens and IOL industries notified the FDA of a UDI labeling strategy being employed for these devices that would
result in an extremely large number of data submissions to the Global Unique Device Identification Database (GUDID). Not only would the volume of
submissions greatly exceed the best estimates previously available to the FDA, but the FDA also learned that many of these submissions would be virtually
identical files. The FDA determined that granting a 1-year extension would be in the best interest of the public health. This additional time will allow
the FDA to work with the affected labelers to develop an approach to ensure that meaningful data will be submitted to the GUDID.
The new UDI compliance date for labelers of these devices is September 24, 2015. The extension applies to the requirements to provide a UDI on the device
label and packages, format dates on the device label and submit data to the GUDID.
Complete FDA notice can be found here:
http://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm410421.htm.
|

|
|

|
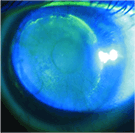
This image represents ring shaped corneal erosion induced by a poorly fit hybrid contact lens. The initial lens was fitted with excessive clearance under
the skirt, that resulted in adhesion after the lens settled back.
We thank Boris Severinsky for this image and we welcome photo submissions from our other readers! It is easy to submit a photo for consideration for
publishing in Contact Lenses Today. Simply visit http://www.cltoday.com/upload/upload.aspx
to upload your image. Please include an explanation of the photo and your full name, degree or title and city/state/country.
^ Back to top
|


|

|

|
OCULAR SURFACE UPDATE
Katherine M. Mastrota,
MS, OD, FAAO
|

|

|

|

|
|
Lessons Learned: Sjo
Sjö is a laboratory test for the diagnosis of Sjögren’s Syndrome in patients with dry eye symptoms.
Briefly, in the office, a small amount of blood is collected on an absorbent card (Whatman card) from the patient via a finger prick. Although a simple
procedure, practice makes perfect!
To ensure an adequate specimen size of good quality, here are important tips:
- Immediately prior to testing, have patients wash their hands well with very warm water, rubbing vigorously to stimulate blood flow to the fingertips.
- A few good arm rotations can send blood to the hands via centrifugal force.
- Massage the patient’s hand, directing blood from the palm to the fingertips prior to sampling.
- Use the lancet to position the finger puncture to the side of the fingertip rather than the middle so that the blood drop does not need to travel across
the finger before it drops.
- Have the patient stand while collecting, keeping the hand below heart level, gently pumping (not milking) the finger to encourage flow.
- Do not touch the finger to the Whatman card to collect the blood drop or overlap drops; allow the blood drop to drop onto the card.
- Collect any extra drops, should they flow, on any clear space on the card space, again, not overlapping drops.
- The blood drops do not have to fit exactly into the printed circles; more importantly, the drops must saturate the card from front to back.
- Since the hands may cool off quickly, a hot cup of water or hot water bottle can be kept nearby, keeping the second hand warm in case it is needed for a
sample.
- Read the package insert thoroughly and carefully for complete sampling instructions.
For more detail on the test procedure, you may view a video here: http://www.mynicox.com/products/sjo-s/.
Extend the power of your practice and offer Sjo point-of-care testing to your dry eye patients.
^ Back to top
|
|
Multifocal Contact Lenses – It Is Important to Know What You Are Dealing With.
Researchers evaluated the power profiles via quantitative deflectometry of a number of soft multifocal contact lens designs to better understand the
distribution of power across the optic zone of the lenses.1 The Nimo TR1504 (Lambda-X, Nivelles Belgium) was used to analyze the power
distribution of Air Optix Aqua Multifocal Low, Medium and High Addition and Focus Progressives multifocal contact lenses. Three lenses of each design were
measured. Results indicated that all multifocal CLs showed a power profile characterized by a change toward more positive power values when aperture sizes
become smaller. The near refractive additions of the lenses under study were +2.61 D, +1.44 D, +1.30 D and +0.30 D for the Focus Progressives, the Air
Optix Aqua Multifocal High, Medium Add and Low Add, respectively. The refractive power of the Focus Progressives did not reach the value of the nominal
distance power until a radial distance of 0.9 mm from the center of the lens. For the Air Optix Aqua Multifocal Low Add the distance nominal power was
reached at a radial distance of 1.5mm from the center of the lens, whereas this occurred at a distance of 1.8mm for the Air Optix Aqua Multifocal Medium
and High Add.
The power distribution of the Focus Progressives is obviously quite different than the more current Air Optix Aqua Multifocal design, regardless of the add
power (high vs. medium vs. low). The Focus Progressives has a much higher add power peak, however it is limited to a very central portion of the optics
whereas the power distribution is much more gradual in the Air Optix Aqua Multifocal design. Add powers were lower than one might expect for the Air Optix
Aqua Multifocal design. This may explain why the use of “pushing plus” on the non-dominant eye is often needed for patients with higher add requirements,
in my experience. The effect of the aperture size on power values can be related to patient’s pupil size. Smaller pupil sizes in these center near aspheric
design multifocal contact lenses will emphasize near vision and can have a negative impact on distance vision performance.
The researchers concluded that the relation between the pupil diameter of the patients and the power profile of these CLs has a crucial implication on the
final distance correction and near addition that these lenses provide to patients. Practitioners should know the power profile of multifocal CLs and also
measure the pupil diameter of each patient in different lighting situations in order to carry out a customized fitting approach. A clear understanding of
the specifics of multifocal contact lens designs can allow the practitioner to most appropriately select lenses that would have the highest probability of
success.
1. Montés-Micó R, Madrid-Costa D, Domínguez-Vicent A, Belda-Salmerón L, Ferrer-Blasco T. In vitro power profiles of multifocal simultaneous vision contact
lenses. Cont Lens Anterior Eye. 2014 Jun;37(3):162-7.
^ Back to top
|

|

|
|
Evaluation of a Novel Eyelid-Warming Device in MGD Unresponsive to Traditional Warm Compress Treatment: An In Vivo Confocal Study.
|
|
The purpose of the study was to evaluate the efficacy and safety of wet chamber warming goggles (Blephasteam) in patients with meibomian gland dysfunction
(MGD) unresponsive to warm compress treatment.
Researchers consecutively enrolled 50 adult patients with low-delivery, non-cicatricial, MGD, and instructed them to apply warm compresses twice a day for
10 minutes for 3 weeks and to use Blephasteam (Laboratoires Thea, Clermont-Ferrand, France) twice a day for 10 minutes for the following 3 weeks. They
considered "not-responders" to warm compress treatment the patients who showed no clinically significant Ocular Surface Disease Index (OSDI) improvement
after the first 3 weeks.
Clinical and in vivo confocal outcome measures were assessed in the worst eye (lower BUT) at baseline, after 3 weeks, and after 6 weeks. Eighteen/50
patients were not-responders to warm compress treatment. These patients, after 3 weeks of treatment with Blephasteam, showed significant improvement of
OSDI score (36.4 ± 15.8 vs. 20.2 ± 12.4; P < 0.05, paired samples t test), increased BUT (3.4 ± 1.6 vs. 7.6 ± 2.7; P < 0.05), and decreased acinar
diameter and area (98.4 ± 18.6 vs. 64.5 ± 14.4 and 8,037 ± 1,411 v. 5,532 ± 1,172, respectively; P < 0.05). Neither warm compresses nor Blephasteam
determined adverse responses.
The authors concluded that eyelid warming is the mainstay of the clinical treatment of MGD and its poor results may be often due to lack of compliance and
standardization. Blephasteam wet chamber warming goggles are a promising alternative to classical warm compress treatment, potentially able to improve the
effectiveness of the "warming approach."
Villani E, Garoli E, Canton V, Pichi F, Nucci P, Ratiglia R. Evaluation of a novel eyelid-warming device in meibomian gland dysfunction unresponsive to
traditional warm compress treatment: an in vivo confocal study. Int Ophthalmol. 2014 Apr 22. [Epub ahead of print]
|
|
|

|
|
A Proud Supporter of

Important Links:
To report adverse contact lens reactions visit:
http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers
Act or associated Contact Lens Rule visit:
https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe;
Unsubscribe; Submit Clinical
Image
Submit news to cltoday@pentavisionmedia.com.
Send your comments and fitting tips to
cltoday@pentavisionmedia.com. Please include your full name, degree or title
and city/state/country.
For more information on Contact Lenses Today including archives of previous
issues, please visit our website at www.cltoday.com.
For the latest articles on contact lenses, important clinical information and helpful
tools related to the contact lens practice visit the Contact Lens Spectrum
website at www.clspectrum.com.
© 2014 All Rights Reserved Contact Lenses Today and CLToday
are registered trademarks of:
PentaVision LLC, 321 Norristown Road, Suite 150, Ambler, PA 19002 | 215-646-8700
© 2014 PentaVision LLC All Rights Reserved.
|

|
|
|













 Plan now to attend the Global Specialty Lens Symposium to be held January 22 – 25, 2015 at Bally's Hotel and Casino in Las Vegas, Nevada. This meeting will
include insightful presentations by international experts in the field, hands-on demonstrations of cutting-edge products and valuable continuing education
credits.
Plan now to attend the Global Specialty Lens Symposium to be held January 22 – 25, 2015 at Bally's Hotel and Casino in Las Vegas, Nevada. This meeting will
include insightful presentations by international experts in the field, hands-on demonstrations of cutting-edge products and valuable continuing education
credits.






