

|
Contact lens care remains vital as ever in the successful wear of contact lenses. We have no doubt learned more and more about contact lens material and lens care compatibility with each new product that is released. Although it has taken significant time, the U.S. Food and Drug Administration (FDA) is acting in this regard and is in the process of proposing new guidance. Some of this guidance has been presented by FDA representatives at two meetings this past year—the Contact Lens Association of Ophthalmologists' meeting in January 2012 and at the ARVO meeting in May 2012. We have asked for the FDA's help in promoting this information to the community through Contact Lens Spectrum, and hope to be able to bring more of this information to you soon. |

Paragon Vision Sciences announced the introduction of NormalEyes 15.5, the only scleral lens design that employs a Dual Axis scleral landing zone. In addition this 15.5mm diameter design utilizes crossover technology combining the patented Proximity Control and Dual Axis technologies also found in Paragon CRT. Dual Axis Technology minimizes lens flexure and ensures rotational stability by providing appropriate sagittal depths in cross meridians. The Dual Axis feature results in predictable centration and provides enhanced comfort, according to the company.
NormalEyes 15.5, the first Proximity Control Technology scleral lens, delivers precise corneal and limbal clearance and allows the eye care professional to control three independent zones. The Dual Axis landing zone allows near-equal circumferential scleral contact and simultaneous control of limbal clearance and edge lift. The patented convex to the eye posterior edge configuration results in predictable conjunctival compression without the possibility of corneal impingement.
The lens is manufactured in Paragon HDS 100 material, laser marked with lens parameters, and is plasma treated. It can be prescribed on normal corneas, corneas with regular astigmatism, keratoconus, Post-LASIK, Post-RK, PKP, PMD and DALK. Two fitting sets can be purchased: the 72-Lens Full Fitting Set or a 21-Lens Starter Set. The lens is manufactured at Paragon's headquarters and distributed worldwide throughout their laboratory network.
For an educational presentation, more information and to view the list distributing laboratories, visit www.paragonvision.com/normaleyes. |
|
On the latest edition of Healthy Vision with Dr. Val Jones, optometrists Jason Pingel and Christi Closson join Dr. Val to talk about how to get the full benefit of contact lens wear and reduce the chances of developing problems that could affect vision and eye health.
In addition to answering commonly asked questions about sleeping in contact lenses, proper techniques for cleaning and storing contact lenses, and the risks of cleaning contacts with any kind of water, the doctors offer some pre-Halloween advice to parents or anyone thinking of purchasing or wearing cosmetic lenses without a contact lens examination and prescription.
In conjunction with the show, to help contact lens wearers better understand how to safely wear and care for their contacts, Johnson & Johnson Vision Care, Inc. has developed a new educational resource, Healthy Vision & Contact Lenses available at www.acuvue.com/press.
The program is supported by Acuvue Brand Contact Lenses. Free podcasts of Healthy Vision with Dr. Val Jones can be found on BlogTalk Radio (www.blogtalkradio.com/healthyvision).
|
|
Art Optical introduces CLASIKcn, a custom GP multifocal lens designed for the growing number of post-LASIK patients struggling with near-vision demands. The lens features front surface, center-near technology specifically designed to provide a full range of simultaneous vision for post-LASIK corneas. CLASIKcn is an option for those finding their surgically induced modified mono effect becoming inefficient as they advance in presbyopia, or for any post-refractive surgery patient encountering presbyopic progression.
The mechanics of the CLASIKcn dual reverse geometry posterior curve system create alignment with both the post treatment area and the non-treated cornea outside of the ablation zone for a comfortable, stable fit; while the strategically placed optic system restores maximum distance, intermediate and near visual function, according to the company.
Featured in Paragon HDS materials, the lens is backed by Art Optical's Risk-Free Signature lens guarantee. Practitioners may choose to fit and design CLASIKcn lenses from corneal topography plus refractive data, or pre/post-surgical Ks plus refractive data, or through the utilization of the CLASIKcn Diagnostic Fitting Set which is available for purchase or through a loaner program.
Complete product details can be found at the company website, www.artoptical.com. |
|
Kingwood, Texas-based optometric alliance, Vision Source, announces the promotion of Bret Davis from National Director of Business Development to Vice President of Business Development.
Prior to his employment at Vision Source, Davis was the President of Briot USA. He joined Vision Source in 2008 and has worked in the optometric industry for 15 years.
|

Aniridia-Related Keratopathy
By William Townsend, OD, FAAO |
|
 |
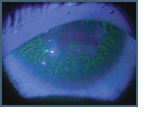 The individual in this photo has aniridia, a rare, progressive condition with multiple ocular and systemic manifestations. Its name denotes iris involvement, in which the iris is deficient or absent, but it also encompasses the cornea, anterior chamber, lens, retina, macula, and optic nerve. The frequency of anirida is reported to be 1:64,000 to 1:100,000. The most common cause is a mutation of the PAX6 gene on band p13 of chromosome 11. Netland et al evaluated the frequency of disorders associated with anirida. They reported the following ocular conditions: nystagmus (83 percent), cataract (71 percent), dry eye (53 percent), glaucoma (46 percent), keratopathy (45 percent), foveal hypoplasia (41 percent), strabismus (31 percent), and retinal disease (5 percent).1
The individual in this photo has aniridia, a rare, progressive condition with multiple ocular and systemic manifestations. Its name denotes iris involvement, in which the iris is deficient or absent, but it also encompasses the cornea, anterior chamber, lens, retina, macula, and optic nerve. The frequency of anirida is reported to be 1:64,000 to 1:100,000. The most common cause is a mutation of the PAX6 gene on band p13 of chromosome 11. Netland et al evaluated the frequency of disorders associated with anirida. They reported the following ocular conditions: nystagmus (83 percent), cataract (71 percent), dry eye (53 percent), glaucoma (46 percent), keratopathy (45 percent), foveal hypoplasia (41 percent), strabismus (31 percent), and retinal disease (5 percent).1
For more on aniridia, its progression and management please see: http://www.clspectrum.com/articleviewer.aspx?articleID=106635.
We welcome photo submissions from our readers! It is easy to submit a photo for consideration for publishing in Contact Lenses Today. Simply visit http://www.cltoday.com/upload/upload.aspx to upload your image. Please include an explanation of the photo and your full name, degree or title and city/state/country.
1. Netland PA, Scott ML, Boyle JW 4th, Lauderdale JD. Ocular and systemic findings in a survey of aniridia subjects. J AAPOS. 2011 Dec;15(6):562-6.
^ Back to top |

|
Written Patient Education in a Thriving Contact Lens Practice
In my August 26th CL Today column, I reported on my husband's friend—an intelligent, rule-abiding, engineer—who reported never receiving written instruction from his practitioner regarding his contact lens wear and care. As such, I sought out the expertise of some of my colleagues, all Diplomates of the Cornea, Contact Lenses and Refractive Technologies section of the American Academy of Optometry with thriving contact lens practices. I wanted to know what they include in their contact lens care informational handout material to help educate patients and improve compliance.
Interestingly, while their forms were different in layout, they contained certain commonalities. These included: how to care for contact lenses, including hand washing; the care system name with specific instruction not to substitute other solutions; advice not to exceed the prescribed wearing time; contraindications against swimming or bathing in contact lenses; symptoms that require an urgent return to clinic, including pain, redness, and blurry vision; practice and emergency phone numbers; patient signatures; and others.
Patients will not remember 100% of what we teach them in clinic verbally, so written documentation goes a long way in reinforcing that education and increasing compliance.
The author would like to thank Drs. Doug Benoit, Clarke Newman, and Susan Resnick for their contributions to this article.
^ Back to top
|
 |  |
 |
OCULAR SURFACE UPDATE
Katherine M. Mastrota, MS, OD, FAAO |
 |  |
 |  |
|
Botulinum Toxin and Dry Eye, Part 4
Installment 1 of botulinum toxin and dry eye discussed the potential complication of dry eye syndrome in patients receiving repeated botulinum toxin injections into the lateral canthal ryhtids (crow's feet).
Omar Ozgur, MD and colleagues contend that repeated neurotoxin chemodenervation of the obicularis oculi leads to a poor blink mechanism, lagophthalmos, scleral show and ectropion that may result in dry eye signs and/or symptoms. Subtle early signs of dry eye syndrome secondary to botulinum toxin injection, Dr. Ozgur holds, can be assessed for via the lid "snap-back" and "distraction" tests.
The snap-back test, which measures muscle tone, is performed by pulling the lower lid inferiorly while the patient looks straight ahead without blinking. Upon releasing, if the lid recovery is not immediate before the next blink, the snap-back test is positive. Documented in seconds, the test is graded on a 1+ to 3+ scale. The lid distraction test measures lid laxity and is performed by pulling the lower lid away from the globe. The distance between the globe and central lid margin is measured and similarly graded in millimeters. Since 2mm is considered a normal result for the distraction test; 6mm or more would be graded at 3+.
In conjunction with patient symptom inquiry, a proposed guideline was developed so that by recognition of the early signs of dry eye syndrome, injections of botulinum toxin can be delayed or discontinued to prevent worsening manifestations of dry eye.
Ozgur O, Murariu, D, Parsa A, Parsa FD. Dry eye syndrome due to botulinum toxin type-a injection: guideline for prevention. Hawaii J Med Public Health. 2012 May (5); 120-123
^ Back to top
|
 |
 |
|
Dry Eye Medication Use and Expenditures: Data from the Medical Expenditure Panel Survey 2001 to 2006
|
Researchers wanted to study dry eye medication use and expenditures from 2001 to 2006 using a nationally representative sample of U.S. adults.
This study retrospectively analyzed dry eye medication use and expenditures of participants of the 2001 to 2006 Medical Expenditure Panel Survey, a nationally representative subsample of the National Health Interview Survey. After adjusting for survey design and for inflation using the 2009 inflation index, data from 147 unique participants aged 18 years or older using the prescription medications Restasis and Blephamide were analyzed. The main outcome measures were dry eye medication use and expenditures from 2001 to 2006.
Dry eye medication use and expenditures increased between the years 2001 and 2006, with the mean expenditure per patient per year being $55 in 2001 to 2002 (n = 29), $137 in 2003 to 2004 (n = 32), and $299 in 2005 to 2006 (n = 86). This finding was strongly driven by the introduction of topical cyclosporine emulsion 0.05% (Restasis; Allergan, Irvine, CA). In analysis pooled over all survey years, demographic factors associated with dry eye medication expenditures included gender (female: $244 vs. male: $122, P < 0.0001), ethnicity (non-Hispanic: $228 vs. Hispanic: $106, P < 0.0001), and education (greater than high school: $250 vs. less than high school: $100, P < 0.0001).
The authors concluded that there was a pattern of increasing dry eye medication use and expenditures from 2001 to 2006. Predictors of higher dry eye medication expenditures included female gender, non-Hispanic ethnicity, and greater than a high school education.
Galor A, Zheng DD, Arheart KL, et al. Dry Eye Medication Use and Expenditures: Data from the Medical Expenditure Panel Survey 2001 to 2006. Cornea. 2012 Jan 6.
^ Back to top |
|
|
 |
A Proud Supporter of

Important Links:
To report adverse contact lens reactions visit: http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers Act or associated Contact Lens Rule visit: https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe; Unsubscribe; Submit Clinical Image
Submit news to news@cltoday.com.
Send your comments and fitting tips to cltoday@wolterskluwer.com. Please include your full name, degree or title and city/state/country.
For more information on Contact Lenses Today including archives of previous issues, please visit our website at www.cltoday.com. For the latest articles on contact lenses, important clinical information and helpful tools related to the contact lens practice visit the Contact Lens Spectrum website at www.clspectrum.com.
© 2012 All Rights Reserved
Contact Lenses Today and CLToday are registered trademarks of:
Springer VisionCare, 323 Norristown Road, Suite 200, Ambler, PA 19002 | 215-646-8700
© 2012 Springer Science + Media. All Rights Reserved.
|
 |
|
|














 The individual in this photo has aniridia, a rare, progressive condition with multiple ocular and systemic manifestations. Its name denotes iris involvement, in which the iris is deficient or absent, but it also encompasses the cornea, anterior chamber, lens, retina, macula, and optic nerve. The frequency of anirida is reported to be 1:64,000 to 1:100,000. The most common cause is a mutation of the PAX6 gene on band p13 of chromosome 11. Netland et al evaluated the frequency of disorders associated with anirida. They reported the following ocular conditions: nystagmus (83 percent), cataract (71 percent), dry eye (53 percent), glaucoma (46 percent), keratopathy (45 percent), foveal hypoplasia (41 percent), strabismus (31 percent), and retinal disease (5 percent).1
The individual in this photo has aniridia, a rare, progressive condition with multiple ocular and systemic manifestations. Its name denotes iris involvement, in which the iris is deficient or absent, but it also encompasses the cornea, anterior chamber, lens, retina, macula, and optic nerve. The frequency of anirida is reported to be 1:64,000 to 1:100,000. The most common cause is a mutation of the PAX6 gene on band p13 of chromosome 11. Netland et al evaluated the frequency of disorders associated with anirida. They reported the following ocular conditions: nystagmus (83 percent), cataract (71 percent), dry eye (53 percent), glaucoma (46 percent), keratopathy (45 percent), foveal hypoplasia (41 percent), strabismus (31 percent), and retinal disease (5 percent).1



