|
I heard some good comments last week about important clinical research topics that are needed to help the field move forward. Obvious topics include continued issues such as safety in contact lens wear, and particularly the issues of the rates and risk factors for infiltrative keratitis, in addition to improving comfort in contact lenses. I would strongly urge you to attend our Global Specialty Lens Symposium meeting in January 2012. Although an obvious emphasis of the meeting is specialty contact lenses, we also cover all other mainstream contact lens-related topics including those mentioned above. If you haven't been to the meeting before, please consider attending. |

Bausch + Lomb (B+L) has expanded its voluntary recall of six lots of Murocel Methylcellulose Lubricant Ophthalmic Solution, USP 1%, only available within the United States. Following the voluntary recall, B+L will discontinue the product entirely.
B+L has chosen to discontinue Murocel Methylcellulose Lubricant Ophthalmic Solution because the company currently has products within its portfolio to treat the same symptoms for which Murocel is indicated. The over-the-counter ophthalmic solution indicated for temporary relief of burning and irritation due to dryness of the eye has been available for use in the United States for more than a decade.
B+L initiated the six-lot recall based on routine stability testing which showed the lots were out of specification for preservative efficacy. There have been no adverse events reported that have been attributed to the product lots being out-of-specification for preservative efficacy. B+L says it is recalling the lots of product in the interest of patient safety.
U.S. retailers and eyecare practitioners were alerted to the recall. B+L is asking consumers who currently have product matching the affected lots to return it to B+L. Consumers who have this product should call the customer service center for instructions on returning and reimbursement between 9 a.m. and 5 p.m. EST Monday through Friday at 1-800-553-5340.
Affected lot numbers and expiration dates are: Lot: 467061, Exp: 09-30-2011; Lot: 487551, Exp: 11-30-2011; Lot: 548051, Exp: 05-31-2012; Lot: 132171, Exp: 08-31-2012; Lot: 136571, Exp: 11-30-2012; Lot: 140181, Exp. 01-31-2013. The UPC Code is 3 24208 28015 7 |
Robert Koetting, OD, who started in a small optometric office with his father and went on to become a leading contact lens practitioner, author and inventor, died Aug. 7 in Bedford, Texas. He was 85.
Dr. Koetting was the third generation of his family to become an eyecare practitioner. His grandfather opened a small optical shop alongside his jewelry store in the 1880s. His father Felix Koetting began his optometric practice in 1921. Dr. Koetting joined him and then opened his own practice in 1962 in St. Louis. While building his successful practice, he became a champion of contact lenses, sharing his practice management ideas with other practitioners.
He was went on to author several books, including Marketing, Managing and Contact Lenses. He was a co-founder of the International Society for Contact Lens Research and the American Society of Contact Lens Specialists, as well as a charter member of the AOA Contact Lens Section.
Dr. Koetting received the Chancellor's Medallion from the University of Missouri St. Louis, the American Optometric Association's Distinguished Service Award and the Eminent Service Award from the American Academy of Optometry.
A private burial will be held in Ste. Genevieve, MO. |

August 31, 2011 is the deadline for submission of papers and posters for the Global Specialty Lens Symposium (GSLS), to be held January 26 - 29, 2012 at the Paris Hotel and Casino in Las Vegas. Papers and abstracts related to presbyopia, keratoconus, corneal topography, post penetrating keratoplasty or related irregular corneal surface, myopia control, orthokeratology and lens care topics are welcome.
New this year is the photo contest. Contestants may submit up to two (2) photographic images in the following anterior segment categories: Contact Lens, Lids, and Cornea/Conjunctiva. Contestants may also submit images obtained utilizing equipment such as OCT, topographers, etc.
Visit www.GSLSymposium.com for more information. Web submissions only.
--ADVERTISING
|
The ARVO Foundation for Eye Research (AFER) announced two new fellowships for AMD and dry eye research projects for investigators younger than age 45. Traditionally, AFER's awards programs have recognized researchers' accomplishments rather than funded their research.
The AFER/Genentech Age-related Macular Degeneration and AFER and Vistakon Dry Eye fellowships are accepting applications until Sept. 15, 2011. Two individuals will receive $40,000 each for work in these specific areas of research.
Both fellowships are open to investigators who are younger than 45 at the application deadline. Fellowships are for a period of one year and must commence within six months of the award notification.
Applications will be reviewed by a committee of ARVO and AFER representatives who are experts in the areas of AMD and dry eye research. Award recipients will be notified Dec. 1, 2011. |
Conforma Contact Lenses has received both ISO 9001:2008 and ISO 13485:2003 certifications, along with Part 1 CMDR SOR 98/282 for its Quality Management System.
The ISO certification was made through BSI Management Systems, an accredited registrar that performs assessments of management systems against requirements of national and international standards for quality.
Conforma Contact Lenses is a custom GP lens manufacturer specializing in proven aspheric lens designs.
^ Back to top |

 |  |
 |
OCULAR SURFACE UPDATE
Kelly K. Nichols, OD, MPH, PhD, FAAO |
 |  |
 |  |
|
Symptoms in Stage 1 MGD
The recent publication of the TFOS-sponsored MGD report provides a clinical algorithm for MGD diagnosis and management (http://www.tearfilm.org/pdfs/TFOS_Mgd_Report_Overview.pdf). Several world-renowned clinicians assisted in the discussions on MGD staging, and after debate consensus was achieved that stage 1 should include patients with slightly abnormal meibomian gland secretions, minimal to no symptoms, and no corneal staining. Taken literally, this would indicate that not even one dot would be present via fluorescein corneal staining, the patient would not present complaining of eye irritation, yet abnormal secretions would be present. Does stage 1 exist in routine clinical practice? In order to truly know, asymptomatic patients would undergo meibomian gland expression—perhaps not a common clinical practice. Yet the looming question remains: if a patient is not symptomatic in the presence of abnormal clinical findings (expression), is treatment warranted? The beauty of new guidelines is that clinicians take the challenge to test the system, and we all look forward to the ensuing discussion.
^ Back to top
|
 |  |
 |
CARE SOLUTION CORNER
Susan J. Gromacki, OD, MS, FAAO |
 |  |
 |  |
|
Tips for Handling Sclerals
Scleral gas permeable contact lenses are becoming an increasingly utilized option for our specialty contact lens patients. Recently, I asked Greg DeNaeyer, OD, FAAO, a Contributing Editor of Contact Lens Spectrum and an experienced fitter of scleral lenses, for his personal recommendations regarding the handling of these large-diameter lenses.
First, he stressed the importance of properly filling the lens prior to application. He recommends filling the entire lens to the edge, not just the central optic zone or "bowl". This is vital to preventing the air bubbles that can commonly enter an under-filled lens upon application. http://www.clspectrum.com/article.aspx?article=105001
To fill the lens, he recommends a 0.9% NaCl inhalation solution supplied in 3 ml. vials. They are over-the-counter and readily available. Although technically still considered off-label, this practice eliminates the possibility of a patient developing a toxicity or hypersensitivity reaction from preservatives. This is especially important since scleral lenses have less tear exchange than smaller-diameter GP lenses; as a result, exposure to a substance behind the lens is enhanced as compared with corneal contact lens wear. Regarding the volume, he prefers the single-use dosage size due to the slight potential for bottle contamination of multiple-use non-preserved solution.
http://www.reviewofcontactlenses.com/content/d/solutions_and_lens_care/c/27236/
http://commons.pacificu.edu/mono/4/#b.mon.tag
^ Back to top
|

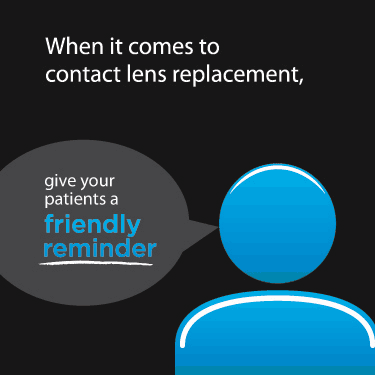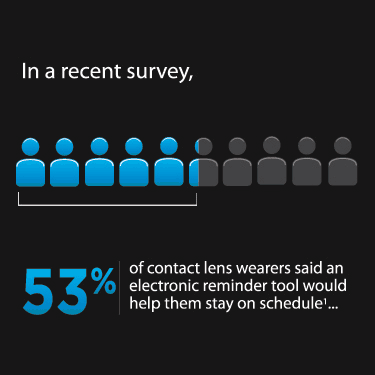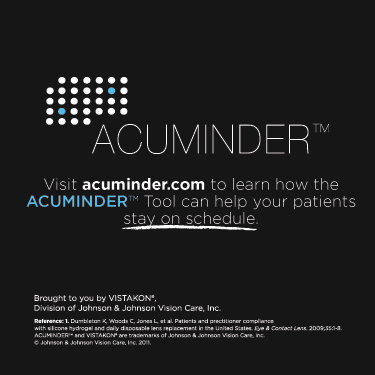
Improving Contact Lens Compliance by Explaining the Benefits of Compliant Procedures
|
|
The goal of the study was to increase compliance with instructions for safe and successful contact lens wear by helping patients understand the advantages and consequences of compliant and non-compliant behavior respectively.
A series of explanations which elucidate the practical and scientific basis for many of the instructions given at lens delivery and aftercare have been prepared as a means of extending patient education beyond simply being instructed on what to do. The summary versions of these explanations have been prepared at an easier level of readability (age 10-12 years) to assist young patients and adults with reading disabilities, including those for whom English is their second language.
Researchers concluded that patients may be non-compliant because they do not understand the practical and/or scientific basis for procedures and the potential consequences of aberrant behavior. Delay in the onset of symptoms associated with non-compliance may allow them to assume that compliance is not important. Explanations that describe why instructions given for lens use are consistent with sustained comfortable and safe lens wear appear to have the potential to strengthen or change patient attitudes toward being compliant. Behavior modification that reduces the prevalence of non-compliance appears likely to also help sustain better contact lens performance and reduce the prevalence of contact lens failure.
These explanations could be modified for use in different practices according to the preferences of individual practitioners and to include new research findings as they become available.
McMonnies CW. Improving contact lens compliance by explaining the benefits of compliant procedures. Cont Lens Anterior Eye 2011
^ Back to top |
|
|
 |
Important Links:
To report adverse contact lens reactions visit: http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers Act or associated Contact Lens Rule visit: https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe; Unsubscribe; Submit Clinical Image
Submit news to news@cltoday.com.
Send your comments and fitting tips to cltoday@wolterskluwer.com. Please include your full name, degree or title and city/state/country.
For more information on Contact Lenses Today including archives of previous issues, please visit our website at www.cltoday.com. For the latest articles on contact lenses, important clinical information and helpful tools related to the contact lens practice visit the Contact Lens Spectrum website at www.clspectrum.com.
Contact Lenses Today and CLToday are registered trademarks of:
Wolters Kluwer Pharma Solutions VisionCare Group, 323 Norristown Road, Suite 200, Ambler, PA 19002 | 215-646-8700
© 2011 All Rights Reserved
|
 |
|
|