

|
As contact lens practitioners, our role is to fit and monitor our patients in contact lenses. However, have you ever stopped to think about the other side of the coin—that is, what it takes to develop a contact lens? Gazing into a crystal ball, what sort of technology do you think is needed to change the dynamic of the contact lens market. As always, we'd love to hear from you on this (jnichols@optometry.uh.edu). |

CooperVision announced that it is expanding its worldwide recall of the Avaira brand product line of contact lenses to include a limited number of lots of Avaira Sphere contact lenses. In continued collaboration with the United States Food and Drug Administration, CooperVision is expanding the recall because it identified certain lots of Avaira Sphere lenses that did not meet its updated quality requirements due to the level of a silicone oil residue. The presence of the residue on Avaira Sphere contact lenses may cause hazy vision or discomfort, severe eye pain or eye injuries requiring medical treatment. Not everyone experiences the same symptoms.
The total number of Avaira Sphere lenses manufactured that are affected by the recall is 6.6M, of which 4.9M were shipped to customers globally. CooperVision intends to replace the recalled product with available Avaira Sphere inventory from lots that are not impacted by this recall action and will also continue to supply Avaira Sphere lenses that are not affected by this recall.
As part of the expanded recall, CooperVision is notifying its customers and requests that eyecare practitioners contact their patients regarding this recall. Communication efforts are focused at eyecare professionals and distributors to effectively reach lens wearers. CooperVision recall efforts include:
- Sending worldwide recall notifications to Avaira Sphere CooperVision customers.
- Issuing a press release via PR Newswire and Globe Newswire regarding the recall of limited lots of CooperVision Avaira Sphere lenses.
- Providing information on the CooperVision website for lens wearers to check if their lenses are impacted (www.coopervision.com/international-recall).
- An information notice has been posted on www.coopervision.com.
- Offering a toll-free consumer hotline (1-855-526-6737)
- Providing retailers and distributors access to patient communications materials.
- Providing support and detailed information to eyecare practitioners to remove recalled lots of Avaira Sphere lenses from the market place.
- Offering customer care resources to answer questions from patients.
|
Unilens Vision Inc. and No. 7 Contact Lens Laboratory Ltd. announced that No. 7 has been named the exclusive distributor in the United Kingdom for the Unilens' C-VUE Advanced HydraVUE family of custom silicone hydrogel contact lenses. No. 7 will also serve as a C-VUE Advanced HydraVUE distributor throughout the European Union Countries.
According to Unilens, C-VUE Advanced HydraVUE custom contact lenses incorporate highly developed lens design technology, including Unilens' patented Toric Multifocal and Multifocal designs and made-to-order Custom Toric and Single Vision options. The C-VUE Advanced HydraVUE presbyopic lens technology allows the eyecare professional to specify the exact add power (for near vision) and zone size.
All four lens options are completely customizable, will be sold exclusively to independent eyecare professionals, and feature a risk-free trial program, advanced silicone hydrogel material and exceptional deliverability. C-VUE Advanced HydraVUE silicone hydrogel lens material is the result of a partnership between Unilens and the new Definitive silicone hydrogel material manufactured by Contamac. |

Plan now to attend the Global Specialty Lens Symposium in January 2012. With an expert international faculty and a CE-accredited agenda, the 2012 GSLS will include insightful presentations by experts in the field, hands-on demonstrations of cutting-edge products as well as scientific papers and posters. Look for more detailed information in future issues of Contact Lens Spectrum and online at www.GSLSymposium.com.
--ADVERTISING |
Dakota Sciences and iCon United Corporation entered into an exclusive agreement allowing iCon to manufacture and distribute the patented SoClear Lens Technology throughout Asia as Master Distributor.
Coming from 40 plus years of GP lens making family history, Drs. Richard and Joseph Wu with their brother Jerry Wu formed iCon United Corporation in Taiwan to bring specialty lens products into the Asia Pacific region. iCon United is working closely with local distributors in countries such as Philippines, Hong Kong, Singapore, Malaysia, China, Japan and Korea to bring the SoClear larger diameter GP lens to eyecare practitioners in those countries.
For further information or questions about SoClear Lens availability in Asia, please email sales@iconunited.com.tw or call +8862.2546.1597. |
On the new edition of Healthy Vision with Dr. Val Jones, two experts, Cristina Schnider, OD, Senior Director, Medical Affairs for Vistakon Division of Johnson & Johnson Vision Care, Inc., and John Ulczycki, Group Vice President — Strategic Initiatives, National Safety Council, join Dr. Val to talk about what happens to eyes in the dark and how patients can take better care of their eyes to improve nighttime driving.
According to a nationwide survey of 515 vision-corrected Americans aged 18 and over, 73% of respondents believe that correcting their vision problems could improve their night time driving, though only 27% have ever consulted an eyecare professional about treatments or products that could improve their vision while driving in the dark*. Dr. Schnider encourages drivers to see their eyecare professional to talk about any problems they are having while driving at night and discusses some of the newer vision correction options currently available.
Healthy Vision with Dr. Val Jones is supported by Acuvue Brand Contact Lenses. Free podcasts of the show can be found in the iTunes Store, BlogTalk Radio (www.blogtalkradio.com/healthyvision) and on http://getbetterhealth.com/healthyvision. A link to the show also can be found at www.acuvue.com/healthyvision.
*Shedding Light on Driving in the Dark, a nationwide survey conducted by Kelton Research on behalf of Road & Travel Magazine and Acuvue Brand Contact Lenses. To view the findings from the survey visit http://www.acuvue.com/pdf/SheddingLightonDrivinginthedarkexecsum.pdf
^ Back to top |

Acute Hydrops
By Melissa Barnett, OD, FAAO, UC Davis Eye Center, Sacramento, CA |
|
 |
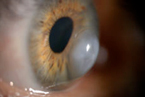 This is a photo of acute hydrops in a patient with keratoconus. This is a photo of acute hydrops in a patient with keratoconus.
We thank Dr. Barnett for this image and welcome photo submissions from our other readers! It is easy to submit a photo for consideration for publishing in Contact Lenses Today. Simply visit http://www.cltoday.com/upload/upload.aspx to upload your image. Please include an explanation of the photo and your full name, degree or title and city/state/country.
^ Back to top |

 |  |
 |
CARE SOLUTION CORNER
Susan J. Gromacki, OD, MS, FAAO |
 |  |
 |  |
|
Recent History of the Alcohol-Based Daily Cleaner
For years, patients and practitioners alike have utilized an isopropyl-alcohol based cleaner for either daily cleaning at home or problem-solving in the office. The primary advantage of this cleaner is its ability to emulsify lipids. It has no additional preservatives, can be used with soft and rigid lenses, may remove some mucin, and provides significant antimicrobial activity.
The most well-known of this category, MiraFlow (CIBA Vision), was discontinued last year. There was also a similar version in a CVS bottle. About six months ago, this too was discontinued. At the present, Walgreens does carry a product, Walgreens Extra Strength Daily Cleaner. Although it is not a private label/generic version of MiraFlow—nor is it manufactured by CIBA (now Alcon)—it does contain the same ingredients: isopropyl alcohol, purified water, poloxamer-407, and amphoteric-10. There is also no data on whether it has the same or similar efficacy profile as MiraFlow.
So, for your patients who require an alcohol-based cleaner, know that one is still available—for now.
^ Back to top
|
 |  |
 |
OCULAR SURFACE UPDATE
Kelly K. Nichols, OD, MPH, PhD, FAAO |
 |  |
 |  |
|
By the Numbers
As I write this, estimates of population based on census data indicate that the world's population currently is 6,973,623,909 people, with over 312 million in the U.S. (updated by the minute at http://www.census.gov/main/www/popclock.html). As the world moves towards 7 billion inhabitants, it is easy to "do the math" relative to the overwhelming burden of worldwide blinding eye disease—including the millions that are vision impaired simply because they cannot get the glasses they need.
However, on a day-to-day basis, our lives, and our practices rarely come into contact with these startling facts (although they should), so we manage what is "close to home." Let's face it, when it comes to practice, a patient's symptomatic complaints influence our management decisions, from contact lenses, to glasses, to diseases such as dry eye and MGD. And based on the sheer number of individuals afflicted by dry eye symptoms across the world, addressing dry eye is an opportunity to make a local difference.
A recent Allergan Dry Eye survey by Harris Interactive demonstrated that close to 70% of US adults who experience one or more symptoms have never visited an eye care professional to treat symptoms. In the U.S., there are 40.6 million people over the age of 65 years, thus 70% translates to 28 million people—likely one of those individuals is sitting in your office right now as a parent, grandparent, or caregiver of a patient—or as a patient. In addition, the survey showed approximately two out of five U.S. adults who visited an eyecare professional to treat their dry eye symptoms stated that they visited more than once before finding relief (19%); or that they still have not found relief (22%), indicating significant unmet need.
So, as "7 billion day" approaches, ask yourself what you can do to act locally, as well as remember to think globally. For more information on the Allergan Dry Eye survey, see http://newsfromaoa.org/2011/11/06/new-allergan-survey-shows-48-have-dry-eye-symptoms/ and for more information on Optometry Giving Sight global initiatives see http://www.givingsight.org/.
^ Back to top
|

|
Decrease in Rate of Myopia Progression with a Contact Lens Designed to Reduce Relative Peripheral Hyperopia: One-Year Results |
|
This group of researchers from the Brien Holden Vision Institute in Sydney, Australia wanted to determine whether a novel optical treatment employing contact lenses to reduce relative peripheral hyperopia can slow the rate of progress of myopia.
Chinese children, aged 7 to 14 years, with baseline myopia between sphere -0.75 to -3.50 D and cylinder </=1.00D, were fitted with the novel contact lenses ( n=45) and followed for 12 months, and their progress compared with a group (n=40) that has similar entry criteria for age, gender, refractive error, axial length, and parental myopia wearing normal, single vision, sphero-cylindrical spectacles.
Upon adjusting for parental myopia, gender, age, baseline spherical equivalent (SphE) values and compliance, the estimated progression in SphE at 12 months was 34% less, at -0.57 D, with the novel contact lenses (95% CI: -0.45 D to -0.69 D) compared with -0.86 D (95% CI: -0.74 D to -0.99 D) for spectacle lenses. For an average baseline age of 11.2 years, baseline SphE of -2.10D, a baseline axial length of 24.6mm, and 320 days of compliant lens wear, the estimated increase in axial length (AL) was 33% less at 0.27 mm (95% CI: 0.22 mm to 0.32 mm) versus 0.40 mm (95% CI: 0.35 to 0.45 mm) for the contact lens and spectacle lens groups, respectively.
The researchers concluded that the 12-month data support the hypothesis that reducing peripheral hyperopia can alter central refractive development and reduce the rate of progress of myopia.
Sankaridurg P, Holden B, Smith E, 3rd, et al. Decrease in Rate of Myopia Progression with a Contact Lens Designed to Reduce Relative Peripheral Hyperopia: One-Year Results. Invest Ophth Vis Sci. 2011 Oct 28. [Epub ahead of print]
^ Back to top |
|
|
 |
A Proud Supporter of

Important Links:
To report adverse contact lens reactions visit: http://www.accessdata.fda.gov/scripts/medwatch/ or call (800) FDA-1088.
To report possible grievances related to the Fairness to Contact Lens Consumers Act or associated Contact Lens Rule visit: https://www.ftccomplaintassistant.gov/.
CLToday Services:
Subscribe; Unsubscribe; Submit Clinical Image
Submit news to news@cltoday.com.
Send your comments and fitting tips to cltoday@wolterskluwer.com. Please include your full name, degree or title and city/state/country.
For more information on Contact Lenses Today including archives of previous issues, please visit our website at www.cltoday.com. For the latest articles on contact lenses, important clinical information and helpful tools related to the contact lens practice visit the Contact Lens Spectrum website at www.clspectrum.com.
Contact Lenses Today and CLToday are registered trademarks of:
Wolters Kluwer Pharma Solutions VisionCare Group, 323 Norristown Road, Suite 200, Ambler, PA 19002 | 215-646-8700
© 2011 All Rights Reserved |
 |
|
|















 This is a photo of acute hydrops in a patient with keratoconus.
This is a photo of acute hydrops in a patient with keratoconus. 


